The extraction of aluminium involves two steps that is purification of bauxite by baeyer s process and electrolysis of alumina. It is the most abundant metal in the earth s crust as it makes up 8 of the crust and it is the third most abundant element after oxygen and silicon.
Draw A Neat Well Labelled Diagram Of Electrolytic Cell For Extraction Of Aluminium Chemistry Shaalaa Com
It involves two steps.
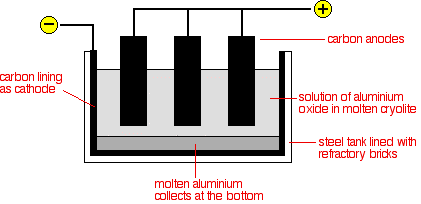
The extraction of aluminium. Bauxite ore al2o3 xh2o is the major source of aluminium till date which is a mixture of hydrated aluminium oxide. The extraction of aluminium from its ore involves following steps. First of all aluminum oxide needs to be in molten form to extract the aluminum ions.
Purification of bauxite by baeyer s process. In the baeyer s process the bauxite ore is heated with concentrated naoh solution under pressure aluminum is purified by leaching method. The temperatures needed are too high to be economic.
Instead it is extracted by electrolysis. Mostly aluminium is extracted from its ore called bauxite. Aluminium is then extracted from aluminium oxide by electrolysis.
Metallurgy of aluminium or extraction process of aluminium from its ore involves various methods. Therefore aluminum oxide is dissolved in molten cryolite. Aluminium oxide has a very high melting point over 2000 degrees celsius.
The science of extracting pure metals by economically effective methods from their ores is called metallurgy. Extracting aluminium aluminium is the most abundant metal on earth but it is expensive largely because of the amount of electricity used in the extraction process. Aluminum oxide however has a high melting point.
The aluminium oxide must be made molten so that the ions can pass through it during electrolysis. Aluminium ore is called bauxite. So instead of trying to melt it the aluminium oxide is dissolved in molten cryolite.
Aluminium is too high in the electrochemical series reactivity series to extract it from its ore using carbon reduction. Aluminium is normally extracted from bauxite ore al2o3. Extraction of aluminium a soft silvery white corrosion resistant metal.
Aluminum is extracted from aluminum oxide by a process called electrolysis. 1 purification of bauxite 2 electrolysis of alumina.
ads
Pages
Search This Blog
Labels
- 1958
- 4 color playing cards
- 4-color suit playing cards
- a place where money is manufactured
- abrasive grinding wheel manufacturers in india
- adoption
- african
- after
- alcohol made from potatoes
- alcohol made from potatoes ireland
- alexander graham bell date of birth
- alexander graham bell date of birth and death
- aluminium foil manufacturing process
- aluminium foil manufacturing process pdf
- amaretto
- amber rings with insects
- annabelle
- antibiotics are produced by
- antibiotics are produced by bacteria or fungi
- are bagels fried or baked
- are bagels made with eggs
- are tortilla chips fried or baked
- armored vans for sale
- armored vans for sale used
- army insert small arms blk 7227
- art sketches of tattoo designs
- attorney
- average number of baseballs used in a game
- baby wipes box cover
- baking soda into washing soda
- beanie baby chocolate 1993
- beanie baby chocolate 1993 value
- beanie baby pvc pellets
- beanie baby pvc pellets vs pe pellets
- beanie baby stuffed animals
- best
- best formula milk for babies in india
- best frozen orange juice concentrate
- best t shirt manufacturers in india
- best type of mouse trap
- best type of mouse trap to use
- best windmill blade design
- big teddy bears for sale
- big teddy bears for sale in sri lanka
- biodegradable plastic bags manufacturing process
- biofuel
- bird cage wire fronts
- black
- bloody
- board
- boat made of bottles
- boat made out of bottles
- boiling point of grain alcohol
- borden eagle brand sweetened condensed milk
- borden eagle brand sweetened condensed milk - 14oz
- born
- braids
- brands of baking powder
- brands of baking powder without aluminum
- build mini jet engine
- building
- building a stirling engine
- building a stirling engine generator
- bullet proof jacket is made up of which material
- bullet proof jacket made of metal
- buy used cowboy boots
- cake shaped like ice cream cone
- camera lens how it works
- can steel be used in microwave
- can you make helium
- can you make helium gas
- cancer
- candles
- capricorn
- carbon fiber recurve bow
- cards
- cast iron foundry project report pdf
- cast of the piano
- cast of the piano film
- cats
- chance
- chemical formula for leather
- chemical formula for white vinegar
- chemical preparation of soap
- chemical properties of aluminum oxide
- chemical reaction of fermentation
- chemical reaction of fermentation process
- chemical structure of acrylic
- chemical structure of acrylic paint
- chemicals used in automotive industry
- chemicals used in making paper
- christmas
- clay based cat litter brands
- clear
- clothes
- coffee beans to coffee
- coffee beans to coffee maker
- cognac made from grapes
- coir and coir products
- commercial wrapping paper rolls
- compatibility
- compatible
- components of a battery
- components of a battery charging system of a wind farm
- components of a heat pump for supplying heated air to a dwelling are shown in the schematic
- components of a heat pump system
- components of a light bulb
- condenser for air conditioner
- consultation
- cooking oil production process
- cooking oil production process pdf
- cost of building a billboard
- cost of building a billboard in kenya
- cost of pregnancy test kit
- cost of pregnancy test kit in dubai
- count ferdinand von zeppelin
- count ferdinand von zeppelin invention
- cow made of butter ted cruz
- cow made out of butter
- crab meat nutritional value
- cramps
- credit
- cruises
- cryolite in aluminium extraction
- daniel hale williams childhood
- dare
- deck
- design
- design for safety ppt
- design patterns for clothes
- development of the pacemaker
- diagram parts of a football
- difference between bleach and chlorine bleach
- difference between fabric softener and dryer sheets
- different types of acrylic nail brushes
- different types of acrylic nails
- different types of baby carriers
- different types of baby carriers pictures
- different types of popsicles
- different types of t shirt collars
- discoloration
- divorce
- do birth control pills prevent pregnancy
- do birth control pills prevent pregnancy in the future
- do ice cream cones have gluten
- does
- does evaporated milk go bad
- does evaporated milk go bad unopened
- does silk come from silkworms
- doll
- dry ice block maker
- drying macadamia nuts at home
- early
- easy
- ekg is used for
- electric tea kettle not made in china
- element used to plate steel to make cans
- elijah mccoy lubricating cup
- engagement
- essential
- evaporated or condensed milk
- evaporated or condensed milk for cheesecake
- example
- examples
- extracting copper from copper oxide
- extracting copper from copper oxide using carbon
- extraction of copper from its ore
- extraction of copper from its ore ppt
- eyes
- faucet washer size chart
- felons
- fiberglass bow and arrow
- finder
- first car to be made
- first car to be made in kenya
- first computer with mouse
- first incandescent light bulb
- first incandescent light bulb invented
- first pair of cowboy boots
- first perfume in the world
- first roller coaster ever made
- fish
- florida
- food
- formula milk for babies
- foundation
- fragrance made in france
- free
- frito lay production process
- from
- furniture
- gain
- ge washing machine parts
- ge washing machine parts for sale
- gift
- golf balls made of
- golf balls made of rubber bands
- gourmet jelly beans flavors
- gram
- greeting card computer programs
- gross
- happy birthday wrapping paper to print
- hcg in pregnancy test
- hcg in pregnancy test kit
- heartburn
- heat
- hemp fabric made in usa
- henry bessemer and william kelly
- high heel shoes for ladies
- high hill shoes for ladies
- high quality silk orchids
- highlights
- home
- homes
- hoover vacuum cleaner attachments
- how aerosol cans are made
- how are arrows made
- how are bricks made
- how are bricks made uk
- how are candles made today
- how are glasses made
- how are glasses made for babies
- how are harps made
- how are incense made
- how are incense sticks made
- how are kayaks made
- how are kettles made
- how are levi jeans made
- how are macadamia nuts grown
- how are macadamia nuts grown and harvested
- how are marshmallow made
- how are polymers created
- how are polymers created from monomers
- how are puzzles cut
- how are puzzles cut video
- how are sponges made
- how are sponges made from trees
- how are synthetic fibres made
- how are thermometers used
- how are zippos windproof
- how atm machine works internally
- how ball bearings are made
- how balloons are made
- how balloons are made video
- how boxing gloves are made
- how chocolate was made
- how chocolate was made in the past
- how did carl friedrich gauss die
- how do barcode scanners work
- how do barcode scanners work with excel
- how do jet planes work
- how do kazoos work
- how do they build bridges underwater
- how do they make butter
- how do they make chalk
- how do they make chex cereal
- how do they make corn chips
- how do they make sugar
- how do they process sugar cane
- how do you build a boat in a bottle
- how do you make a bowling ball
- how do you make a bowling ball hook
- how does a gas pump work
- how does a gas station pump work
- how does a rubik's cube work
- how does a rubix cube work
- how does carbon fiber work
- how does cognac taste
- how does cognac taste like
- how does the spinning jenny work
- how does the spinning jenny work step by step
- how hockey sticks are made
- how it's made cement
- how it's made plywood
- how it's made vodka
- how many bales of cotton in a module
- how many laces on a football
- how many laces on a nfl football
- how many pedals on a harp
- how much do cinder blocks weigh
- how much do concrete blocks cost
- how much does a baseball bat weigh
- how much does a baseball bat weigh in grams
- how much does a bowling pin cost
- how much does a build a bear cost with clothes
- how much instant coffee to use
- how playing cards are made
- how tattoos were done in the past
- how they build bridges
- how they make butterfingers
- how they make gum
- how they make gummies
- how they make sugar cubes
- how to bake a pretzel
- how to bake corn chips
- how to build a model roller coaster
- how to build a model roller coaster out of popsicle sticks
- how to build an armored vehicle
- how to can ketchup
- how to color mirror glass
- how to colour dry pasta
- how to draw a bridge over a river
- how to draw a bridge over a river step by step
- how to dry pasta without a rack
- how to extract oil from plants
- how to extract oil from plants using distillation
- how to extract oil from the ground
- how to liquefy helium
- how to make a bow string
- how to make a bulletproof vest out of cardboard
- how to make a bulletproof vest out of duct tape
- how to make a cardboard washing machine
- how to make a celtic harp
- how to make a kaleidoscope without mirrors
- how to make a mold for cement statues
- how to make a one size fits all hat smaller
- how to make a pen cap fly
- how to make a pen cap launcher
- how to make a satellite receiver
- how to make a skyscraper
- how to make a skyscraper in little alchemy 2
- how to make a tattoo design
- how to make a tattoo design online free
- how to make a violin bow
- how to make an oxygen tank
- how to make an oxygen tank in escapists 2
- how to make bird cage at home
- how to make bird cage at home in tamil
- how to make boomerang
- how to make boomerang video
- how to make bubble gum recipe
- how to make calcium carbide
- how to make calcium carbide cannon
- how to make chopsticks
- how to make chopsticks with forks
- how to make custom rubber stamps
- how to make denim fabric
- how to make denim fabric flowers
- how to make hair colour at home
- how to make hair colour with coffee
- how to make homemade dentures
- how to make inline skates
- how to make inline skates more comfortable
- how to make iron magnetic
- how to make led at home
- how to make led lights at home
- how to make lux soap
- how to make lux soap in factory
- how to make matches at home
- how to make nes coffee
- how to make perfume from scratch
- how to make rammed earth walls
- how to make rubber stamp
- how to make rubber stamps with cricut
- how to make ship inside bottle
- how to make soy milk from soybeans
- how to make violin bow at home
- how to make washing soda from baking soda
- how to manufacture a food product
- how to manufacture a food product uk
- how to manufacture paint
- how to manufacture paint using chemicals
- how to mirror glass
- how to prepare bricks
- how to prepare bricks for painting
- how to preserve soya milk
- how to preserve soya milk for days
- how to process honey
- how to process honey from a beehive
- how to produce instant coffee
- how to produce wind energy
- how to produce wind energy at home
- how to remove sensormatic tag
- how to sew a tuxedo jacket
- how to size field hockey stick
- how to size hockey stick
- how to weave hemp
- how to weave hemp necklace
- how was cotton made
- how was cotton made in the industrial revolution
- how was gum made
- how was gum made in the 1800s
- how was the umbrella made
- how was the umbrella made in ancient china
- how we get silk
- how we get silky hair at home
- how were coins made in ancient times
- how were coins made in medieval times
- human blood pressure psi
- hurt
- ice cream cone shaped cake
- ideas
- images of an mri machine
- images of an open mri machine
- impact
- informative
- ingredients in air fresheners
- ingredients in charmin toilet paper
- insert small arms blk 7227
- instrument like a flute
- instrument that looks like a flute
- invention of credit card
- invention of credit card fargo
- inventions of the light bulb
- inventor of traffic signal
- iron ore manufacturing process
- iron ore pellets manufacturing process
- jobs
- joint
- kennel
- key features of a credit card
- kittens
- large globes on stands
- large round bird cage
- large round bird cage with stand
- lava lamp black and white
- lava lamp black and white clipart
- lava lamp not bubbling
- led light bulb manufacturers
- led light bulb manufacturers in india
- letter
- lg fuzzy logic washing machine parts
- life
- list
- lube oil base stock
- lube oil base stock production in petroleum refinery
- lubricating oil manufacturing process
- lubricating oil manufacturing process ppt
- m and m blue packet
- m and m price
- m and m price list
- make a bow string jig
- make a paper boomerang
- make a paper boomerang that comes back
- make vodka at home
- make vodka at home easy
- makeup
- making
- making fiber optic cable
- making of rubber stamp
- making of rubber stamp near me
- making sugar from beets
- male
- manufacturers of jet engines
- manufacturing process of liquid detergent
- manufacturing process of pesticides
- many
- mascara made out of
- mascara made out of bat poop
- material for covering sofas
- material used in microwave oven
- materials to make soap
- materials used in electronics
- meaning
- medium
- methods of extracting natural gas
- microbial production of vinegar
- microbial production of vinegar ppt
- milk to curd process
- milk to curd process time
- model car kit companies
- movie
- much
- museum
- music sheets to print
- music sheets to print out
- names of fizzy drinks
- names of low dose birth control pills
- negative
- noir
- nutritional value of spam
- nutritional value of spam lite
- objectives
- organizations
- ouija
- outline of a girl tattoo
- outline of a tattoo
- ovulation
- pacifiers made in usa
- painted wooden ironing boards
- palm
- paper production process steps
- parts of a baby stroller
- parts of a bird cage
- parts of a football
- parts of backhoe loader
- parts of binoculars diagram
- parts of electric iron box
- parts of iron box
- parts of jcb backhoe loader
- parts of suspension bridge
- parts of the harp
- parts of the harpsichord
- pcb printed circuit board
- pcb printed circuit board definition
- pennyweight
- people
- period
- perms
- pet plastic bottle manufacturers
- pet plastic bottle manufacturers in malaysia
- pick
- pictures of the element silver
- pinball machine parts names
- planed wood cut to size
- plants
- plastic bags manufacturing process
- plastic lawn mower blades
- pocket watch case parts names
- pocket watch parts names
- pop up book card
- pop up book card template
- porcelain how to make
- porcelain vases made in china
- pregnancy
- pregnant
- preparation of ethyne from calcium carbide
- preparation of ethyne from calcium carbide equation
- print out sheet music
- printable sheet music
- printed circuit board construction
- private selection gourmet jelly beans flavors
- probiotics
- procedure of making wine
- process of making cooking oil
- process of making jute
- process of making jute fibre from jute plant
- process of manufacturing a car
- process of refining silver
- process of refining silver by fire
- process of salt production
- product from crude oil
- product made from crude oil
- production of sodium hypochlorite
- production of sodium hypochlorite by electrolysis
- production of soy milk
- production of toilet paper
- production process of dairy milk chocolate
- production process of vodka
- products made from flax
- products made from flax fibres
- products with sodium bicarbonate
- profit
- propane from natural gas
- pros
- push
- quiz
- quotes
- radio flyer wagon red paint
- ragdoll
- raw material for plastic bottle manufacturing
- raw materials for candle making
- raw materials for candle making philippines
- raw materials for concrete
- raw materials for concrete blocks
- real
- recipes
- red clay bricks manufacturing process
- red wine making process at home in hindi
- red wine making process flowchart
- reflective paint for road signs
- remedies
- rent
- rings
- roller skates with rubber wheels
- roller skates with rubber wheels uk
- rolling in manufacturing process
- salicylic acid to aspirin
- salicylic acid to aspirin class 12
- scalp
- scientific name for cheese
- self
- serve
- shade
- sheep to wool process
- signs
- simple printed circuit board
- singers
- sketches of tattoo designs
- skin
- smelting of iron in a blast furnace
- smelting of iron in a blast furnace involves which of the following processes
- soccer ball throwing machine
- soccer ball throwing machine for sale
- social
- software
- solar cells are used to
- solar cells are used to make
- solar powered room heater
- solar powered room heater uk
- sour
- soy sauce how to make
- stages of wool production
- steam iron with base
- steel making process flowchart ppt
- stick making supplies ireland
- stool
- stories
- stroke
- stuffed animals ty beanie baby
- sunflower seeds from plant
- sweet
- symptoms
- system
- t shirt manufacturing process
- t shirt manufacturing process flow chart
- t shirt print machine price
- t shirt print machine price in bangladesh
- t shirt printing steps
- tasting
- tattoo
- tattoos
- tea bag making machine
- tea bag making machine amazon
- teddy bear making process
- teddy bear stuffed toy
- teddy bear stuffed toy price in philippines
- test strips for sugar in urine
- tetra pack juice box
- tetra pack juice box price
- the best ice skates
- the best ice skates for beginners
- the extraction of aluminium by hall-heroult process purified is mixed with to
- the extraction of aluminium storyboard
- the first piano invented
- the first radio ever made
- the ink spots wikipedia
- the introduction of antibiotics such as penicillin
- the making of hot dogs
- the process of making a car
- the process of making a career choice begins with
- the process of making chocolate from cocoa beans
- the process of making ice cream
- the process of making ice cream in factories
- the very first roller coaster ever made
- thermos vacuum insulated mug
- thermos vacuum insulated travel mug
- things made out of beer cans
- thrive
- tinted lenses for eyeglasses
- tools needed to make sushi
- tools to measure blood pressure
- treatment
- tree
- trees
- tubal
- ty penguin stuffed animal
- types of ic and their functions
- types of ic and their functions pdf
- types of screws and bolts
- types of screws and bolts pdf
- use of quartz in watches
- use of silk fabric
- used commercial ice cream machine
- used commercial ice cream machine for sale
- used commercial popcorn machine for sale
- used double door refrigerator
- used double door refrigerator for sale
- used fire hoses for sale
- uses for cat litter buckets
- uses for cat litter containers
- uses of brass and bronze
- uses of brass and bronze jss2
- uses of optical telescope
- vehicle
- vendo soda machine parts
- vertical wind turbine manufacturers
- vintage half frame glasses
- vintage half frame reading glasses
- virgo
- visa
- vitamin d manufacturing process
- volume of us coins
- washing machine moves around
- washing machine moves around when spinning
- water soluble markers crayola
- wear
- weeks
- weight
- what
- what are barbies made out of
- what are bats made of
- what are beam bridges made out of
- what are beam bridges used for
- what are chalkboards made of
- what are circus peanuts made out of
- what are composite bats made of
- what are compound bow limbs made of
- what are condoms made of
- what are condoms made of besides latex
- what are each guitar strings called
- what are erasers made of
- what are faucets made of
- what are guitar strings called
- what are heating coils made of
- what are ice cream cones made of
- what are kites made of
- what are kites made of today
- what are led bulbs
- what are led bulbs used for
- what are liquid crystals made of
- what are noodles made of
- what are peanuts made out of
- what are pickles made of
- what are pickles made out of
- what are pool noodles made of
- what are roller coasters made of
- what are syringes made of
- what are teeth made from
- what are the different parts of the nail
- what are the different parts of the nails and their functions
- what are the ingredients in soy sauce
- what are titaniums uses
- what can oxygen be used for
- what can propane be used for
- what chemicals are in spray paint
- what crayons are made of
- what did lord kelvin do
- what do astronauts wear on their head
- what do you need to make t shirts
- what do you need to make t shirts at home
- what does cognac mean
- what does cognac mean in french
- what does licorice come from
- what does titanium make
- what does toothpaste contain
- what does toothpaste contains acid or base
- what goes into dog food
- what happens when aspartame is heated
- what is a baling machine
- what is a boom on a crane
- what is a natural magnet
- what is a natural magnet name the chemical formula of compound present in it
- what is a typewriter ribbon
- what makes a blimp fly
- what makes a metal magnetic
- what makes natural gas
- what makes natural gas a clean and green fuel class 8
- what metal are paper clips made of
- what plant does licorice come from
- what sound do bells make
- what to do in cognac france
- what to use for temporary tattoos
- what was the first cereal
- what was the first cereal made by kellogg's
- what was the first telephone made of
- what was the first telephone made out of
- what wood are bows made of
- what year was the first tv invented
- what year was the tv invented
- what's mascara made of
- what's the chemical formula for sugar
- whats
- wheat flour mill plant layout
- wheat flour mill plant manufacturers in india
- when did m and m come out
- when was felt invented
- when was the cassette invented
- when was the flute first made
- when was the ink pen invented
- when was the mop invented
- when was the portable cassette player invented
- when was tinfoil invented
- when were inline skates invented
- when were juice boxes invented
- when were robots invented
- where are marlboro cigarettes made
- where are singer sewing machines made
- where are singer sewing machines made now
- where are towels made
- where did eli whitney live
- where did skateboarding originate
- where do pens come from
- where does bowling come from
- where does brandy come from
- where does polystyrene come from
- where does silk comes from
- where does silver come from
- where does silver come from wikipedia
- where does vitamin c come from
- where does vitamin c come from naturally
- where mercury comes from
- where to buy food grade dry ice
- where to buy food grade dry ice near me
- where to put nicotine patches
- where to put nicotine patches on body
- where was eli whitney from
- where was hacky sack invented
- where was pizza made
- where was pizza made originally
- where was spaghetti invented
- where was the jukebox invented
- whirlpool washing machine gearbox price in india
- whirlpool washing machine gearbox price list
- whites
- who invented charcoal briquettes
- who invented electricity before benjamin franklin
- who invented electricity benjamin franklin
- who invented plastic wrap
- who invented the gas mask in 1914
- who invented the mercury barometer
- who invented the mercury barometer quizlet
- who made rubber bands
- who made the first map
- who made the first map of the moon
- who makes degree deodorant
- who makes the best crystal glassware
- who used the telephone when it was first invented
- who was gabriel fahrenheit
- who was michael faraday
- who was michael faraday role model
- why are fizzy drinks fizzy
- why are wind turbines used
- why was chocolate invented
- why was the tv made
- why was the violin made
- will duct tape stick to fabric
- wine
- with
- wonderful
- work
- worms
- wrapping paper to print
- ww2 baby gas mask
- ww2 baby gas mask for sale
- x ray film material
- x ray spex glasses
- yang
- year
- ying
- yogurt makers for sale
- your
Featured Post
quotes from it's a wonderful life movie
It's a Wonderful Life Quotes, Movie quotes – Movie. . “It's a Wonderful Life” Quotes 22 quotes more on this quote ›› “- Claren...

